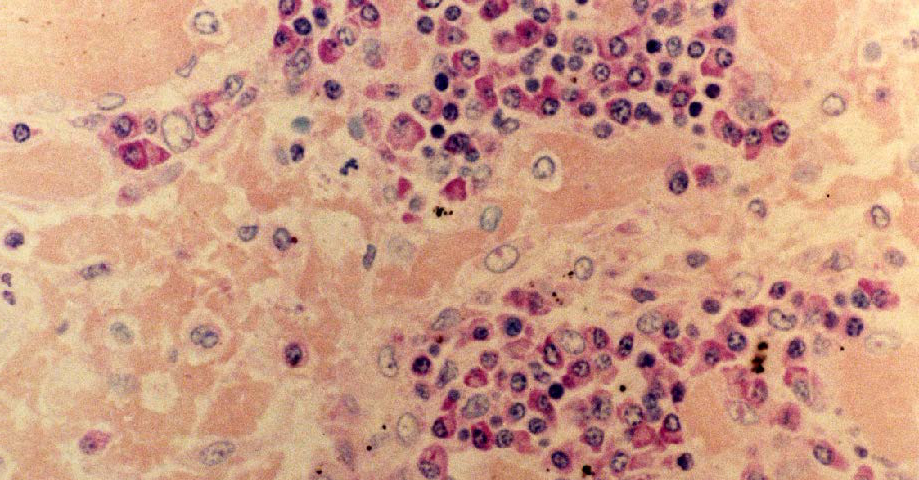
Human lymph node, Methyl green-Pyronin stained, x25 objective
Sections from LR White embedded tissue have been used successfully for immunocytochemistry within both the light microscope and electron microscope fields. This demonstrates quite clearly that the visualisation steps of the immunocytochemical procedure will penetrate the resin and react with the tissue antigens if they have been preserved in the tissue.
As with immuno-localisations, the key factor is whether or not the tissue antigen has survived fixation, processing and embedding in such a form as to be recognisable to the specific antibody. This is difficult to predict with certainty, but some antigens have been shown to be highly resistant whilst others are fickle even in unfixed frozen sections.
Much interest has been centred on using immunocytochemistry to detect protein hormones and the various classes of immunoglobulin and generally these classes of antigen have proved resistant to alteration both in processing to paraffin-wax and to LR White Resin.
It is the special hydrophilic nature of LR White that allows immunochemicals to permeate the supporting resin and reach its sites of binding. No resin pre-treatment is necessary, or indeed possible, to facilitate this penetration.
We have been successful with LR White blocks only when they have been thermally cured, probably because when accelerator-curing the resin the exotherm produced is sufficient to damage the integrity of the tissue antigen. Some workers have also reported that a slight under-cure of LR White, for example at 55°c for 20 to 24 hours aids subsequent penetration of antisera, and have obtained good results without deviating from the standard polymerisation procedure.
If the particular antigen under consideration has already been localised in paraffin-wax sections, then a trusted fixation regime will be established and should be adhered to. For those approaching the problem for the first time, there is an extensive bibliography available regarding fixation for immunocytochemistry, much of it contradictory, and a reference list is provided as some guidance. The rule of thumb seems to be to avoid glutaraldehyde and perhaps use an acid rather than a neutral fixative, however, there are many conflicting and strongly held views on the topic.
Similarly, the need to digest via enzyme sections prior to reaction is fraught with controversy and may indeed be linked to the fixation regime chose. We have used both protease type VII and trypsin type II for good effect on our neutral buffered formalin-fixed material.
If frozen, dewaxed or etched, epoxy resin samples that are used for immuno-staining the tissue are not surrounded by a supporting matrix when they are being reacted. When using LR White sections, the resin is still intact and therefore diffusion to the sites of reaction must occur prior to reaction of antisera with antigen. For this reason, we have found it necessary to use antisera at approximately 10x the concentration that would work on dewaxed sections. The exact titre of each antibody will depend upon its source and how well it has been stored, but we may have used many anti-immunoglobulins at about a 1 in 10 dilution.
For the same reason, the antibody stages of the reaction often benefit from a longer incubation time. Up to 2 hours at room temperature or overnight at 4°C in a moist chamber may be used.
Various immunoperoxidase techniques have given results on LR White tissue sections, including the peroxidase-antiperoxidase complex method (PAP) (Sternberger, 1970), the hapten sandwich technique (Jasani et al, 1981), and the indirect peroxidase method. The Avidin-biotin-peroxidase complex method of Hsu has not been successful in our hands with LR White embedded material, probably due to the molecular size of Avidin. As fairly strong antibody concentrations are required, a highly sensitive method of detection is to be preferred and for this reason, the PAP or hapten sandwich techniques are more suitable than a two-layer indirect peroxidase reaction.
Any technique where the sections are subjected to hydrogen peroxide solutions twice during staining is likely to tend to lift sections from the slides. We have found that poly L lysine (MW 350,000) is an excellent adhesive for immunocytochemical work. Care should be taken to dry sections onto slides very thoroughly in an oven rather than a hot-plate at 60°C for two hours. This step should not have any effect on the antigenicity of the tissue as it will already have spent 20-24 hours at 60°C during polymerisation.
It is clear that no 'standard' immunohistochemical staining regime can be cited as there are so many variables, but a 'typical' regime is described below for general guidance.
PAP procedure for LR White sections (3μm)
| 1. Block Endogenous Peroxidase with 1% Phenylhydrazine Hydrochloride in P.B.S (optional) | 30 mins each |
| 2. Wash in P.B.S | 1 x 5 mins, and 2 x 5 mins at 37°c |
| 3. 0.1 Trypson in 0.1% CaCl2 (aqueous) | 20 mins |
| 4. Wash in ice-cold distilled water | 10 mins |
| 5. 2% Goat serum in P.B.S | 20 mins |
| 6. 1° Antibody (approx 1:10 dilution) | 2 hours at 37°c or overnight at 4°c *Usually Dako or Nordic |
| 7. Wash in P.B.S | 10 mins |
| 8. Goat rabbit antibody (approx 1:20 dilution) | 2 hours at 37°c or overnight at 4°c |
| 9. Wash in P.B.S | 10 mins |
| 10. Goat anti-rabbit antibody (approx 1:20 dilution) | 2 hours at 37°c or overnight at 4°c |
| 11. Wash in P.B.S | 10 mins |
| 12. Wash in Tris HCL pH 7.6 | 10 mins |
| 13. DAB/H2O2 (Graham and Karnovsky) | 15 mins |
| 14. Wash in distilled water | |
| 15. Counterstain as required | |
| For immunocytochemistry LR White must be thermally cured and not accelerator cured. | |





